HighVolumeHDF® as standard.
FX high-flux and FX low-flux dialyzers
Key features
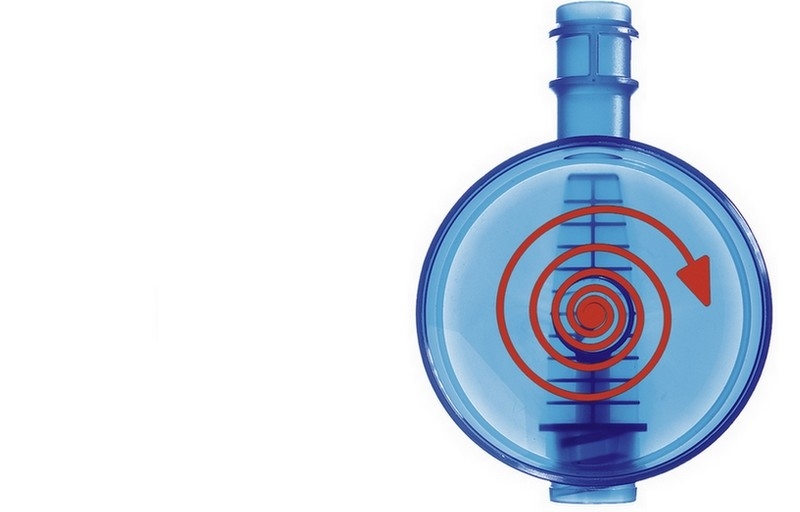
Radial dialyzate flow
- The pinnacle structure of the polypropylene housing ensures a uniform dialyzate flow around the entire fiber bundle
- A high packing density of the fiber bundle and a special wavy fiber structure to avoid dialyzate channeling
- Combined these features enable the constant performance of all FX dialyzers
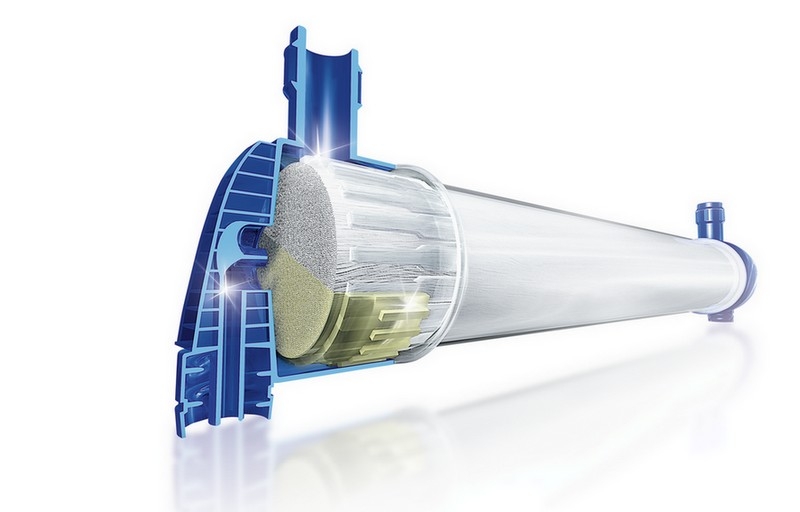
Optimum fiber dimensions
- The reduced inner diameter and wall thickness of the fiber increase the internal filtration and minimize diffusive resistance
- A significant increase of both the diffusive and convective clearances is therefore achieved, allowing the efficient removal of a broad spectrum of uraemic toxins
Technology
Helixone® — the advanced polysulfone membrane
- Nanotechnology membrane fabrication procedures (Nano Controlled Spinning Technology, NCS™) provide Helixone® with a highly-defined pore structure and distribution at the innermost, separating region of the membrane1,2
- Unlike conventional pores which were rugged and uneven in shape, the pores at the inner layer of the Helixone® membrane are smooth and cylindrical
- This reduces the resistance of the molecules when travelling through the pores and allows for enhanced removal
Helixone® has been specially designed to meet the demands of both low-flux and high-flux therapies:
- More even distribution of pores
- Estimated increased average pore size of 1.8 nm (low-flux) and 3.3 nm (high-flux)
- Increased performance per unit of surface area
INLINE steam sterilization – purity ensured
No chemical residuals. Low rinsing volumes. Lower costs.
INLINE steam sterilization – the benefits
- Highly pure, sterile and pyrogen-free dialyzers without any potentially harmful residuals from sterilization
- Biocompatibility of membranes remains unaffected from sterilization
- Optimized use of resources due to low rinsing volumes: only 500 mL is required
- Dry dialyzers with minimized risk of contamination due to microbial growth
Performance data
FX low-flux dialyzers
| FX low-flux dialyzers | FX 5 | FX 8 | FX 10 | |
|---|---|---|---|---|
| Ultrafiltration coefficient (ml/h x mmHg) | 8 | 12 | 14 | |
| Clearance: QB: (200ml/min) | ||||
| Urea | 180 | 191 | 193 | |
| Creatinine | 165 | 178 | 181 | |
| Phosphate | 141 | 160 | 170 | |
| Vitamin B12 | 88 | 107 | 121 | |
| Clearance: QB: (300ml/min) | ||||
| Urea | 228 | 254 | 261 | |
| Creatinine | 200 | 225 | 231 | |
| Phosphate | 164 | 194 | 210 | |
| Vitamin B12 | 94 | 120 | 138 | |
| Clearance: QB: (400ml/min) | ||||
| Urea | 293 | 303 | ||
| Creatinine | 252 | 260 | ||
| Phosphate | 213 | 233 | ||
| Vitamin B12 | 126 | 146 | ||
| The in vitro performance data were obtained with QD = 500ml/min: QF = 0ml/min; T=37°C (ISO8637) The ultrafiltration coefficients were maintained using human blood, Hct = 32%, protein content 6% |
||||
| Effective surface area (m²) | 1.0 | 1.4 | 1.8 | |
| Blood filling volume (ml) | 54 | 74 | 95 | |
| Membrane material | Helixone® | |||
| Housing material | Polypropylene | |||
| Potting compound | Polyurethane | |||
| Sterilization method | INLINE steam | |||
| Application | HD | |||
FX high-flux dialyzers
| FX high-flux dialyzers | FX 40 | FX 50 | FX 60 | FX 80 | FX 100 |
|---|---|---|---|---|---|
| Ultrafiltration coefficient (ml/h x mmHg) | 20 | 33 | 46 | 59 | 73 |
| Clearance: QB: (200ml/min) | |||||
| Urea | 170 | 189 | 193 | 197 | |
| Creatinine | 144 | 170 | 182 | 189 | |
| Phosphate | 138 | 165 | 177 | 185 | |
| Vitamin B12 | 84 | 115 | 135 | 148 | |
| Inulin | 54 | 76 | 95 | 112 | |
| Clearance: QB: (300ml/min) | |||||
| Urea | 250 | 261 | 276 | 278 | |
| Creatinine | 210 | 230 | 250 | 261 | |
| Phosphate | 201 | 220 | 239 | 248 | |
| Vitamin B12 | 130 | 155 | 175 | 192 | |
| Inulin | 81 | 104 | 125 | 142 | |
| Clearance: QB: (400ml/min) | |||||
| Urea | 303 | 362 | 331 | ||
| Creatinine | 262 | 287 | 304 | ||
| Phosphate | 248 | 272 | 284 | ||
| Vitamin B12 | 167 | 190 | 213 | ||
| Inulin | 109 | 133 | 152 | ||
| The in vitro performance data were obtained with QD = 500ml/min: QF = 0ml/min; T=37°C (ISO8637) The ultrafiltration coefficients were maintained using human blood, Hct = 32%, protein content 6% |
|||||
| Effective surface area (m²) | 0.6 | 1.0 | 1.4 | 1.8 | 2.2 |
| Blood filling volume (ml) | 32 | 53 | 74 | 95 | 116 |
| Membrane material | Helixone® | ||||
| Housing material | Polypropylene | ||||
| Potting compound | Polyurethane | ||||
| Sterilization method | INLINE steam | ||||
| Application | HD/HDF/HF | ||||
FX hemodiafilters
| FX hemodiafilters | FX 600 | FX 800 | FX 1000 |
|---|---|---|---|
| Ultrafiltration coefficient (ml/h x mmHg) | 52 | 63 | 75 |
| Clearance: QB: (200ml/min)QF: (0ml/min) | |||
| Urea | 196 | 198 | |
| Creatinine | 184 | 190 | |
| Phosphate | 180 | 184 | |
| Vitamin B12 | 141 | 149 | |
| Inulin | 101 | 110 | |
| Clearance: QB: (300ml/min) QF: (75ml/min) | |||
| Urea | 284 | 289 | 290 |
| Creatinine | 262 | 271 | 280 |
| Phosphate | 254 | 262 | 269 |
| Vitamin B12 | 199 | 209 | 211 |
| Inulin | 150 | 161 | 164 |
| Clearance: QB: (400ml/min) QF: (100ml/min) | |||
| Urea | 351 | 361 | 364 |
| Creatinine | 313 | 328 | 343 |
| Phosphate | 301 | 313 | 325 |
| Vitamin B12 | 229 | 241 | 244 |
| Inulin | 172 | 185 | 188 |
| The in vitro performance data were obtained with QD = 500ml/min: T=37°C (ISO8637) The ultrafiltration coefficients were maintained using human blood, Hct = 32%, protein content 6% |
|||
| Effective surface area (m²) | 1.5 | 1.8 | 2.2 |
| Wall thickness / inner diameter (µm) | 35/210 | ||
| Blood filling volume (ml) | 97 | 118 | 138 |
| Membrane material | Helixone® | ||
| Housing material | Polypropylene | ||
| Potting compound | Polyurethane | ||
| Sterilization method | INLINE steam | ||
| Application | HD/HDF | ||
Related content
1 Bowry, S.K.: Nano-controlled membrane spinning technology: Regulation of pore size, distribution and morphology of a new polysulfone dialysis membrane. In Hemodialysis Technology (eds: Ronco, C., La Greca, G.) Contributions to Nephrology, Vol. 137: 85-94 (2002)
2 Ronco, C., Nissenson, A.R.: Does nanotechnology apply to dialysis? Blood Purification 19: 347-352 (2001)