Medical device regulation (MDR)
Regulation (EU) 2017/745

EU MDR requirements
Fresenius Medical Care is committed to ensuring that all our medical devices meet the applicable MDR requirements in accordance with the respective transitional timelines.
Many questions arise concerning the MDR, regarding:
- Its impact on the continuous availability of Fresenius Medical Care products
- Details on its applicability for customers and users of our medical devices
Due to the extent and complexity of the new MDR, the new requirements are being implemented in accordance with dedicated implementation programmes and controlled by senior management in close coordination with our notified body.
The following Q&A section will provide relevant information on the MDR implementation by Fresenius Medical Care, and its consequences for customers and users of our medical devices.
Questions and answers
Does Fresenius Medical Care fulfil the MDR requirements?
Conformity of Fresenius Medical Cares quality management system with the applicable MDR requirements was assessed and confirmed during external audits by the notified body in 2019. Since then, we are subject to regular surveillance audits regarding MDR conformity.
An equally important prerequisite for obtaining the MDR certification is the product verification in terms of conformity with the requirements of the MDR. After successful assessment by the notified body, the first product categories has been included in the MDR certification scope. The number of products conforming with MDR will constantly increase over the next years in line with the MDR transition provisions.
The respective EU certificate pursuant to the MDR that has been issued by the notified body will be updated accordingly with the respective product categories.
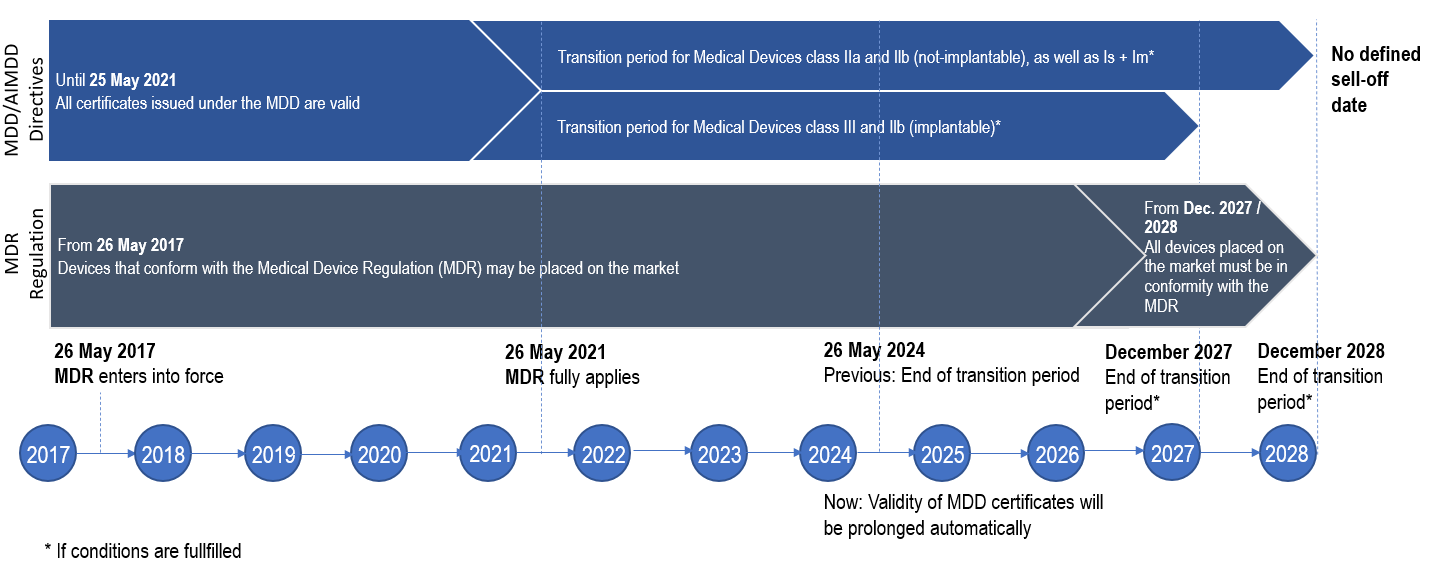
What are the transition timelines from the former Medical Device Directive (MDD) to the MDR applicable for Fresenius Medical Care?
For the general timelines related to MDR transitional provisions see the illustration.
For specific risk classes of medical devices, stricter timelines may apply in accordance with the applicable regulations.
However, the MDR timelines are subject to review and possible modification as deemed necessary by the EU Commission. Prominent examples:
- Due to the COVID-19 crisis the MDR date of application has been postponed by the EU Commission by one year to 26 May 2021 (Regulation (EU) 2020/561)
- An additional amendment of the MDR defined as Regulation (EU) 2023/607 was published in 2023, so the transitional timelines related to class IIa and IIb (not-implantable) and Is+Im Medical Devices are postponed to December 2028 and for class III and IIb (implantable) to December 2027 (if specific conditions are fulfilled). Additionally the previous definded sell-off date for MDD devices was abolished.
When will all affected Fresenius Medical Care products conform with the new MDR requirements?
Our medical devices will be placed on the EU market in conformity with the applicable EU requirements during the legally defined transition periods from the former MDD to the MDR.
Until end of December 2027 / December 2028 (depending on the classification of the Medical Device), products which have been certified under MDD can under certain conditions still be placed on the market after the MDR fully applies, as long as their EC certificates are valid.
Accordingly, Fresenius Medical Care will place both MDD and MDR certified medical devices on the market during this period and, until end of the respective transition periods (see section „MDR Transition timelines“, the Fresenius Medical Care products with MDD certification will gradually be certified according to MDR. Adaptations to the FME portfolio may become necessary in this phase.
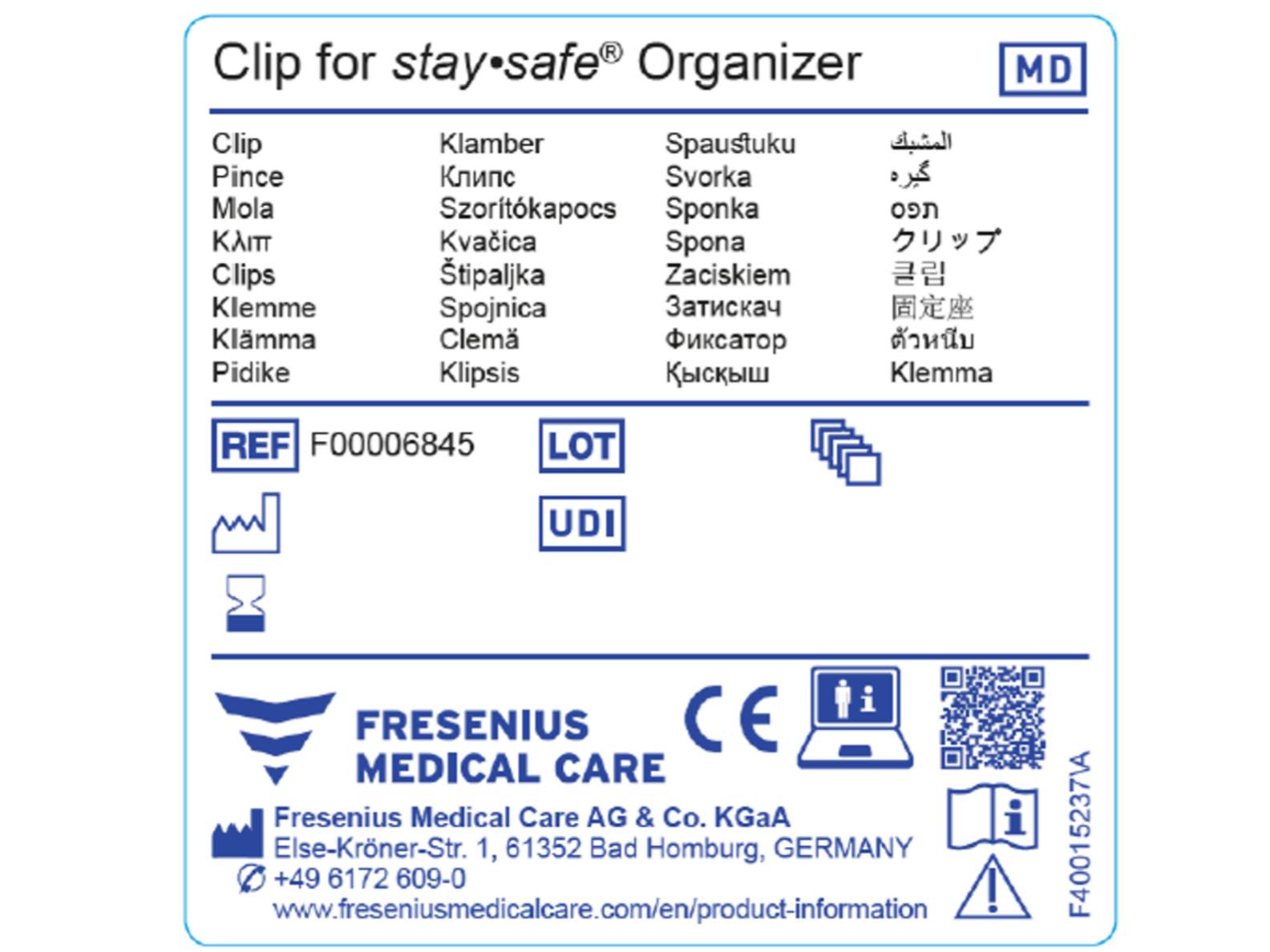
Which changes in labelling are expected for MDR certified products?
MDR certified products require a Unique Device Identifier (UDI). The UDI is a series of numeric or alphanumeric characters that allows the identification of a specific device on the market. Specific symbols are needed for MDR certified products, examples are shown in the first illustration. The UDI code on MDR conform devices does not replace the article number, it is for product identification and traceability during the complete life cycle of the product*.
Please find an example for an MDR conform generic sample label in the second illustration.
What extent of product traceability must customers ensure regarding our medical devices after implementation of the UDI labelling requirement?
MDR certified products require a Unique device identifier (UDI). The UDI is a series of numeric or alphanumeric characters that allows the identification of a specific device on the market.
The relevant applicable obligations for customers are described in EU Commission factsheets (see link section below*) for
- Unique device identifier (UDI)
- Authorized representatives, distributors, and importers
- Healthcare professionals and health institutions
What is the relevance of the EUDAMED database?
The European Database on Medical Devices (EUDAMED) is a database managed by the EU Commission for the storing of information about medical devices.
The aim of EUDAMED is to strengthen market surveillance by providing competent authorities with fast access to information on manufacturers, authorized representatives, medical devices, certificates and vigilance data, to share information on clinical investigation data, as well as to contribute to a uniform application of regulatory requirements, in particular in relation to registration requirements.
All manufacturers and all medical devices to be placed on the EU market will be registered in EUDAMED. Manufacturers (like Fresenius Medical Care) have to ensure that all data provided to EUDAMED are correct and up-to-date. Data includes product related data, data from different market surveillance reports, and data concerning the registered economic operator.
Fresenius Medical Care has to take care of the fulfillment of the requirements regarding EUDAMED registration and reporting in accordance with the legally defined timelines.
Download of instructions for use and declaration of conformity
For MDR certified Fresenius Medical Care products, instructions for use (IFU) will be provided online, in addition to the printed version. The instructions for use and the declaration of conformity (DoC) of MDR certified products can be found here.
Background information on the MDR
Information and factsheets from the EU Commission
To find the following documents do a quick search for:
- Regulation (EU) 2017/745 (MDR)
For other links see:
- EU Commission – Medical devices sector
- Factsheet UDI (as PDF)*
- Q&A: Application of regulation on medical devices
Information and factsheets from other sources
Industrial associations and public authorities for general information on the MDR:
*Factsheets by EU Commission for UDI, for Authorized Representatives / Distributors / Importers, and for Healthcare Professionals by EU Commission