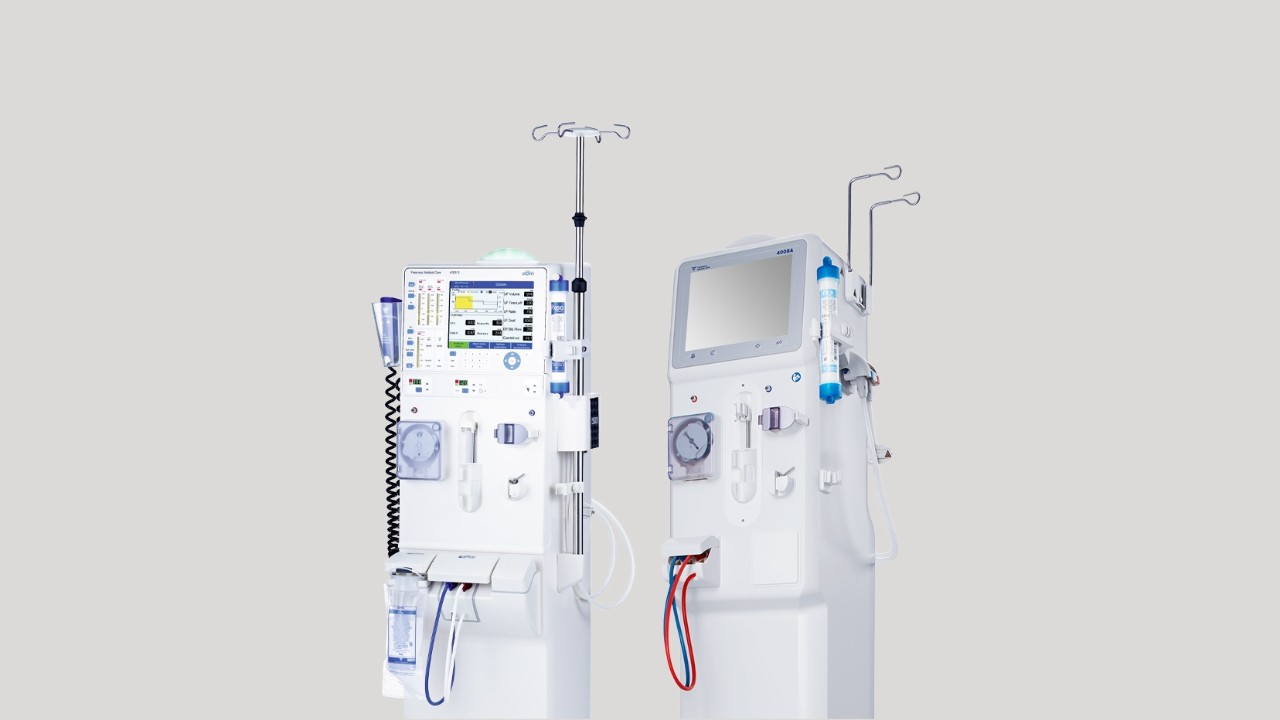
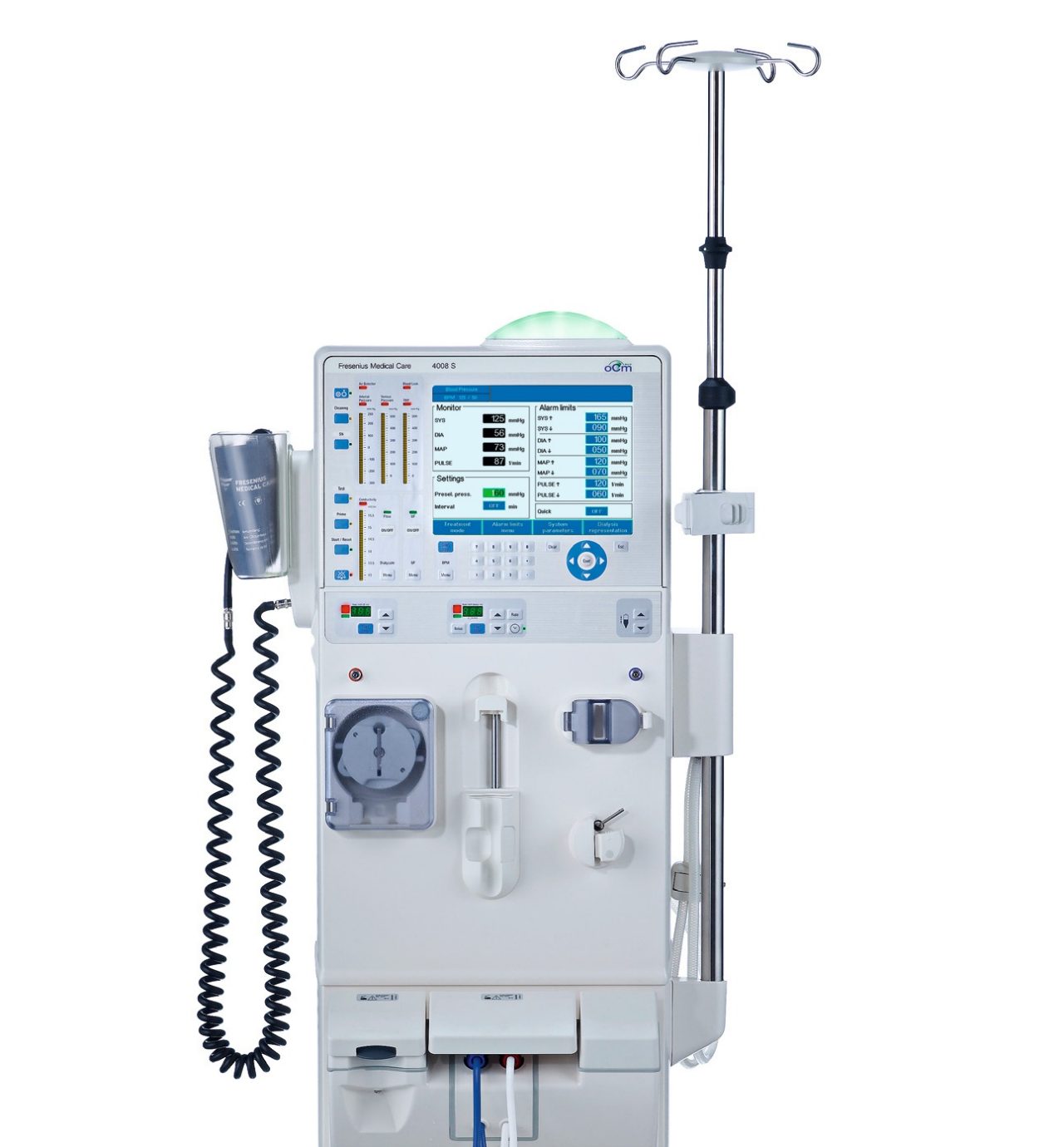
4008 series hemodialysis machines
- Container
- Container
4008S*
The 4008S provides the following functionalities:
- Online assessment of dialysis efficiency and dialysis dose with Online Clearance Monitor (OCM)
- Ultrapure dialysis fluid with DIASAFEplus
- Hygienic dry bicarbonate concentrate supply with bibag
- Improved sustainability via dialysate, concentrate, water and energy savings with AdaptedFlow*
- Integrated measurement of vital parameters with Blood Pressure Monitoring (BPM)
- Optional data management with Therapy Data Management System (TDMS)
- Intuitive and easy handling
*Feature is not available in all countries
4008A
Reliable safety and handling features
Enables high-quality treatments – suggested medical benefits
Contact our local representative for more study results.
1. Sitter T, Bergner A, Schiffl H. Dialysate related cytokine induction and response to recombinant human erythropoietin in haemodialysis patients. Nephrol Dial Transplant 2000;15:1207-1211.
2. Fresenius Medical Care. Unpublished internal data on file.
3. Ledebo I. and Ward R.A., Dialysis – History, Development and Promise, World Scientific Publishing (2012); Chapter 5N: 481-489.
4. Jofré R, Rodriguez-Benitez P, López-Gómez J, Pérez García R.. Inflammatory syndrome in patients on hemodialysis. J Am Soc Nephrol 2006;17:274-280.
5. Lederer SR, Schiffl H. Ultrapure dialysis fluid lowers the cardiovascular morbidity in patients on maintenance hemodialysis by reducing continuous microinflammation. Nephron 2002;91:452-455.
6. Furuya R et al. Ultrapure dialysate reduces plasma levels of beta2-microglobulin and pentosidine in hemodialysis patients. Blood Purif 2005;23:311-316.
7. Scarpioni R et al. Dialysis-related amyloidosis: challenges and solutions. Int J Nephrol Renovasc Dis 2016;9:319-328.
8. Shafi T et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis 2010;56:348-358.
9. Liu X, Dai C. Advances in understanding and management of residual renalfunction in patients with chronic kidney disease. Kidney Dis 2017;2:187-196.
10. Schiffl H, Lang SM, Fischer R. Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Nephrol Dial Transplant 2002;17:1814-1818.
11 Canaud et al. Cellular interleukin-1 receptor antagonist production in patients receiving on-line haemodiafiltration therapy. Nephrol. Dial. Transplant.: 16, 2181-2187, 2001.