Medical responsibility

We provide holistic dialysis care for patients worldwide.
Fresenius Medical Care is organized around the needs of people living with kidney disease, with broad expertise in comprehensive patient care. We are grounded in science. Our clinical experts, researchers, scientists, mathematicians, and caregivers are focused on our vision to create a future worth living for patients, worldwide, every day. By assuming medical responsibility, we continue to lead the way in transforming the industry. At our core, we are driven to translate science into everyday clinical practice that benefits our patients and generates sustainable value for the health care systems we operate within.
Global Medical Office
Our patients’ well-being is our top priority. To continuously deliver on our commitment to delivering safe, high-quality care to patients with chronic illnesses, it is important that we share our interpretations of clinical science and medical practice patterns globally. For this reason, we established the Global Medical Office, which is part of our network that promotes scientific and medical progress worldwide. The Global Medical Office is led by our Global Chief Medical Officer, who is also a member of the Management Board. Key findings of the Global Medical Office are reviewed by dedicated committees. They are published on a regular basis and shared with the medical community.
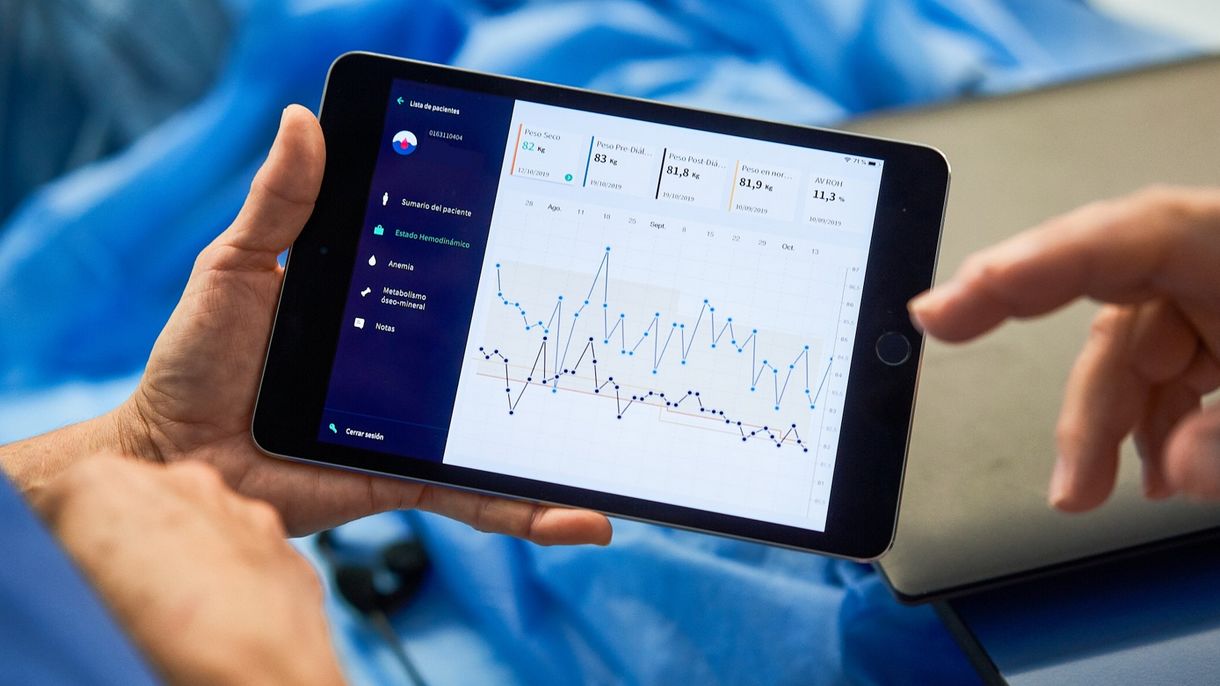
The GMO works to harness the full potential of our global, vertically integrated approach to achieve the best clinical outcomes for our patients, their families, and the payor community. This office is tasked with evaluating coordinated data from clinical science research and medical practice on a global basis to improve treatment outcomes. This includes facilitating cooperation and knowledge transfer across the entire Fresenius Medical Care network.
Our Global Annual Medical Report
Download the full report
Click here to request a hard copy of the 2021 Annual Medical Report.
Therapy of choice
One of the key pillars of our growth is addressing our patients’ changing needs. Many patients would rather be treated at home so that they can lead a life as normal as possible, instead of receiving regular treatment at a dialysis clinic for several hours, several days a week. Patients want a more informed choice and options beyond in-center hemodialysis that fit their life circumstances. By acquiring NxStage in 2019, we expanded our product portfolio with an innovative technology for home dialysis. This enables us to offer our patients greater flexibility and more individual treatment. Home dialysis improves our patients' quality of life.
However, not every patient chooses home therapy or is in the medical condition to do so. Patients may choose or require different modalities throughout their treatment journey:
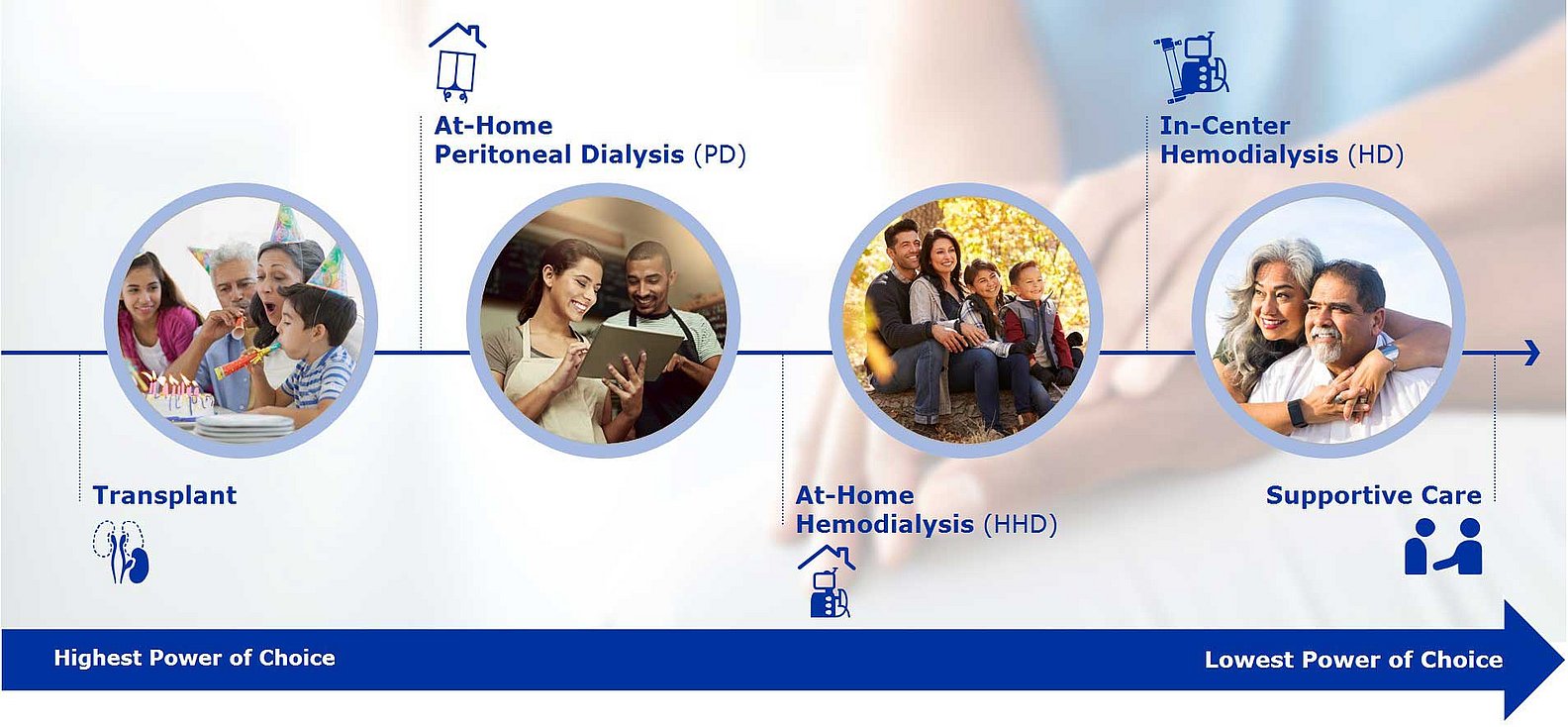
Our goal is to provide every patient the right care at the right time and in the environment they prefer. Read more about different dialysis treatment options here.
Product safety and quality
We aim to develop safe and high-quality products for patients. With our network of 42 production sites in around 20 countries, we control the procurement, production, distribution, and supply of renal and multi-organ therapy products. We manage the quality and safety of our product business over the entire product life cycle, from design and development to operation and application.
Our Global Quality Policy outlines our commitment to product and service quality. The policy covers our obligation to comply with relevant regulations and maintain environmentally sound and efficient operations. It is the basis for regional quality manuals and further policies covering responsibilities, training, risk assessments, and audits. The Management Board is regularly informed about our global quality performance.
Our operations are subject to extensive governmental regulation in almost every country in which we operate. To fulfill our commitment to product quality and safety while complying with relevant regulatory requirements, we have embedded our safety and quality processes in comprehensive quality management systems. This means that products must comply with safety and quality standards concerning product development, manufacturing, their use in clinics, customer training, and complaint handling.
To help use determine the effectiveness of our quality systems, we regularly carry out internal audits at each of our production sites. We assess our quality and management systems against internal and regulatory standards. All audit findings are documented and escalated depending on their criticality, and are used to determine and implement appropriate corrective and preventive measures.
Our plants are also subject to regular external quality audits and reviews in accordance with local requirements. Audits are carried out by notified bodies and authorities, including the U.S. Food and Drug Administration (FDA), the German Ministry of Health and other applicable national health authorities.